Is Hydrogen More Electronegative Than Oxygen
No youve got it backward. Since there are two hydrogen atoms in water molecule there will be a total of 2δ charges in a single water molecule.
Which Is More Electronegative Bromine Or Iodine Quora
They have a positive end and a negative end.
. Droplets of water is due to cohesion. A water molecule is made. Since oxygen is significantly more electronegative than hydrogen oxygen atoms have a much stronger attraction to shared electrons than hydrogen atoms have.
On a same period electronegativity increases from left to right so oxygen is more electronegative than carbon. Why do hydrogen and oxygen form polar covalent bonds within water molecules. Oxygen is more electronegative than hydrogen.
Short Quiz True or False Polarity of water is due to the more electronegative hydrogen and less electronegative oxygen. SO option B is correct 10. Can help me out with my main question what makes Hydrogen more electropositive.
Electronegativity is one of the most useful concepts in chemistry. Since the electrons are in the second shell the electrons start out close to the nucleus. The larger number of protons in an oxygen atoms nucleus allows it to have a greater pull of electron density when its part of a molecule compared to hydrogen.
R-O- is less stable due to I effect of the alkyl group. Oxygen is more electronegative than hydrogen. The oxygen atom is more electronegative it is better than hydrogen at attracting electrons because it has more positively charged protons in its nucleus and this makes it slightly more negative.
In H 2 0 the more electronegative oxygen atom is more negative and the end with the hydrogens is more positive. Hydrogen has a molecular mass of 1 where as an oxygen atom has a molecular mass of 16 so oxygen is heavier than. Which of the following is a correct statement about the atoms in water H201.
Answers 1 A Akshay. Alcohol is weaker acid than water because OH- ion is more stable than R-O- ion. Oxygen is highly electronegative than hydrogen hence oxygen will pull the shared electron pairs in the covalent bond between O and H more towards it an with this oxygen will get a partial negative charge called δ.
In the C-O bond oxygen is more electronegative than carbon so oxygen will have a partial negative charge and carbon a partial positive charge. Which of the following statements correctly identifies and describes the type of covalent bond found between oxygen and hydrogen in water molecules. Oxygen is more electronegative than hydrogen so oxygen pulls the shared paired of electrons towards it.
Carbon is not there unless you are talking about some obscure alternative series. IvanNeretin yeah i noticed it would be great if Dr. Hydrogen is less electronegative than some metals and more electronegative than many others.
A water molecule is made up of. Hydrogen is more electronegative than oxygen generating a partial negative charge near. So sulphur is less electronegative than oxygen.
Therefore ionic bond is stronger than hydrogen bond. In carbonyl group oxygen is more electronegative than carbon and hence the bonding electrons are attracted more towards oxygen. Electronegativity of O is 35 and electronegativity of H is 21 THus there is large electronegativity difference between the two and electronegativity of O is more than H due to which it is polar.
Two hydrogen atoms 2 xx H. 19 views Answer requested by Praveen Upadhyay Related Answer Harsh Pandey. Alcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element.
So being oxygen more electronegative than hydrogen the electrons in a water molecule H₂O or H - O - H are more strongly attractet by the oxygen atoms which means that the electrons will spend more time closer to oxygen atoms than to hydrogen atoms. Acidity of alcohols increases with increase in the stability of acid anion. This creates the partial negative and positive charges on oxygen and carbon respectively.
Consequently the hydrogen atoms are unable to hold the electrons near to them and become slightly more positive. Hydrogen has a positive. Hydrogen bond is a weak electrostatic force of attraction between the hydrogen and most electronegative atoms such as F ON.
The oxygen atom is bonded to each of the two hydrogen atoms via a single bond which as you know is a chemical bond in which two electrons called electrons are. These partial charges act as two poles and hence the bond. Water can make hydrogen bond with its own molecule only.
Attractions form between opposite partial charges. Oxygen has a higher electronegativity 35 than Hydrogen 22 so the electrons are drawn more to the Oxygen than Hydrogen. A polar covalent bonds because hydrogen is more electronegative than oxygen.
Fluorine has the highest electronegativity. There is a partial positive charge on each hydrogen atom and two partial negative charges on the oxygen atom in a water molecule. Water is an electronegative molecule.
Multiple Choice O Hydrogen has a partial positive charge oxygen has a partial negative charge Hydrogen has a partial negative charge oxygen has a partial positive charge Water has an overall positive charge Water has an overall. The positive charge pulls the electrons closer to the nucleus. This unequal sharing of electrons and bent shape results in a polar molecule.
A bonding pair will experience more attraction from the oxygens nucleus than from nitrogens and so the electronegativity of oxygen is greater. The electrons spend more time around the oxygen atom because oxygen is more electronegative than hydrogen. What has more mass hydrogen or oxygen.
Hydrogen bonding is stronger than covalent bonding ionic bonding. View the full answer. Fluorine40 chlorine32 carbon26 as the electronegativity increases when we move along a period so electronegativity of fluorine is highest than carbonhydrogen.
Hydrogen bond is an a. In other words water molecules are polar. However the bonding electrons in the sulphur are further from the nucleus and so the attraction is lessened.
One oxygen atom 1 xx O. Because oxygen is more electronegative than hydrogen. Oxygen has a positive charge of 8 due to having 8 protons.
In case of water molecule which is composed of hydrogen and oxygen. Lattice energy of the ionic bonded compounds are greater than the hydrogen bonded compounds. Surface tension is due to adhesion.
Fluorine has the highest electronegativity. Atomic size which is related to how close the nucleus is to the outer bonding electrons must lso be considered in some cases.
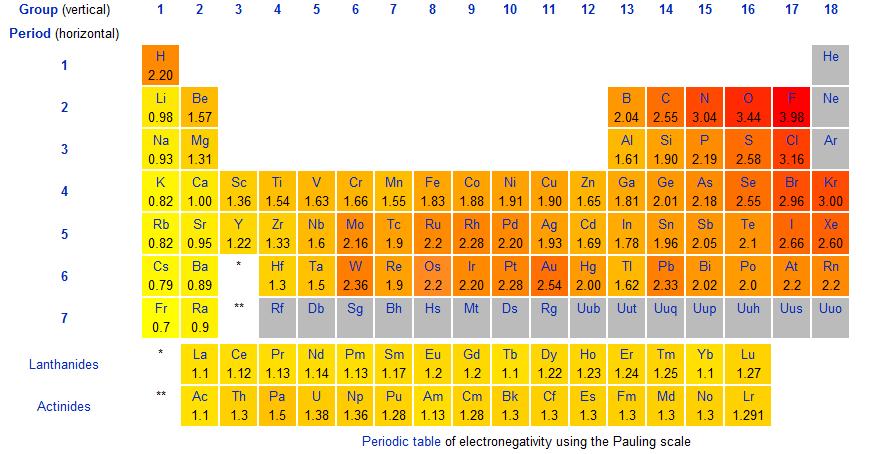
What Is Electronegativity Definition Chart And Trends
Why Is The Electronegativity Of Nitrogen High Quora
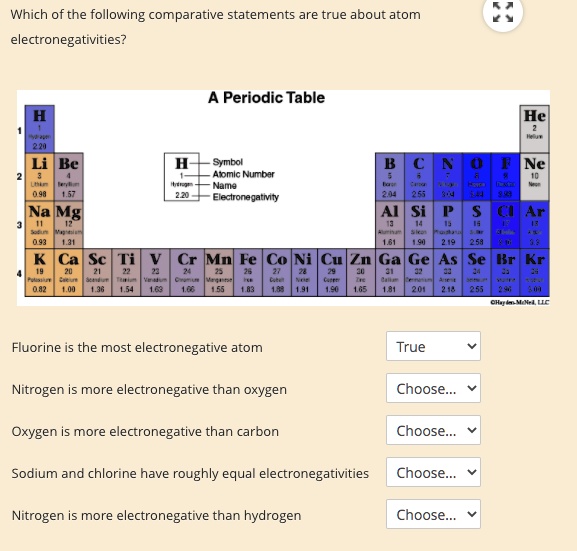
Solved Which Of The Following Lom Parative Statements Are True About Atom Electronegativities Periodic Table He Be Symbol Apmic Numbar Nama Electronegatmty Net Na Mg Ar Derar Ca Sc Mn Co Ni Cu

Primary And Secondary Bonds Hydrogen Bond Covalent Bonding Water Molecule
Why Is The Electronegativity Value For Hydrogen So High Quora
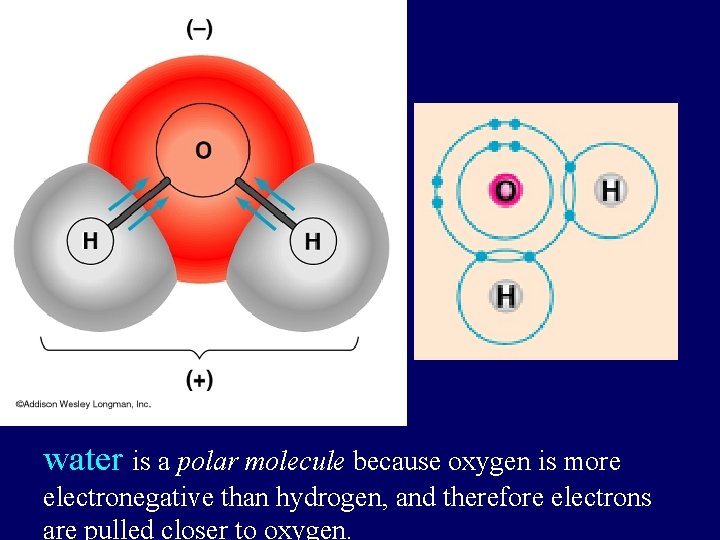
Atom The Smallest Unit Of Matter The Helium

Unixporn Chemistry Computer Technology Paypal Gift Card

Why Boiling Point Of Alcohol Is Higher Than Ether Hydrogen Bond Molecular Boiling Point

Glucopyranose Organic Chemistry Books Physical Chemistry Organic Chemistry

Study Buddies Posts Tagged Yourstudygeek Study Notes Notes Inspiration School Organization Notes

Properties Of Water Water Is Polar Oxygen Is More Electronegative Than Hydrogen Creates Partial Charges At The Hydrogen Atoms A Partial Ppt Download

If Oxygen Is More Electronegative Than Hydrogen Shouldn T It Be Slightly Positive And Hydrogen Be Slightly Negative In H2o Quora

Water A Level Notes Water Molecule Molecules Teaching Chemistry
Which Is More Electronegative Oxygen Or Nitrogen Quora
Why Is O More Electronegative Than Cl And How Quora
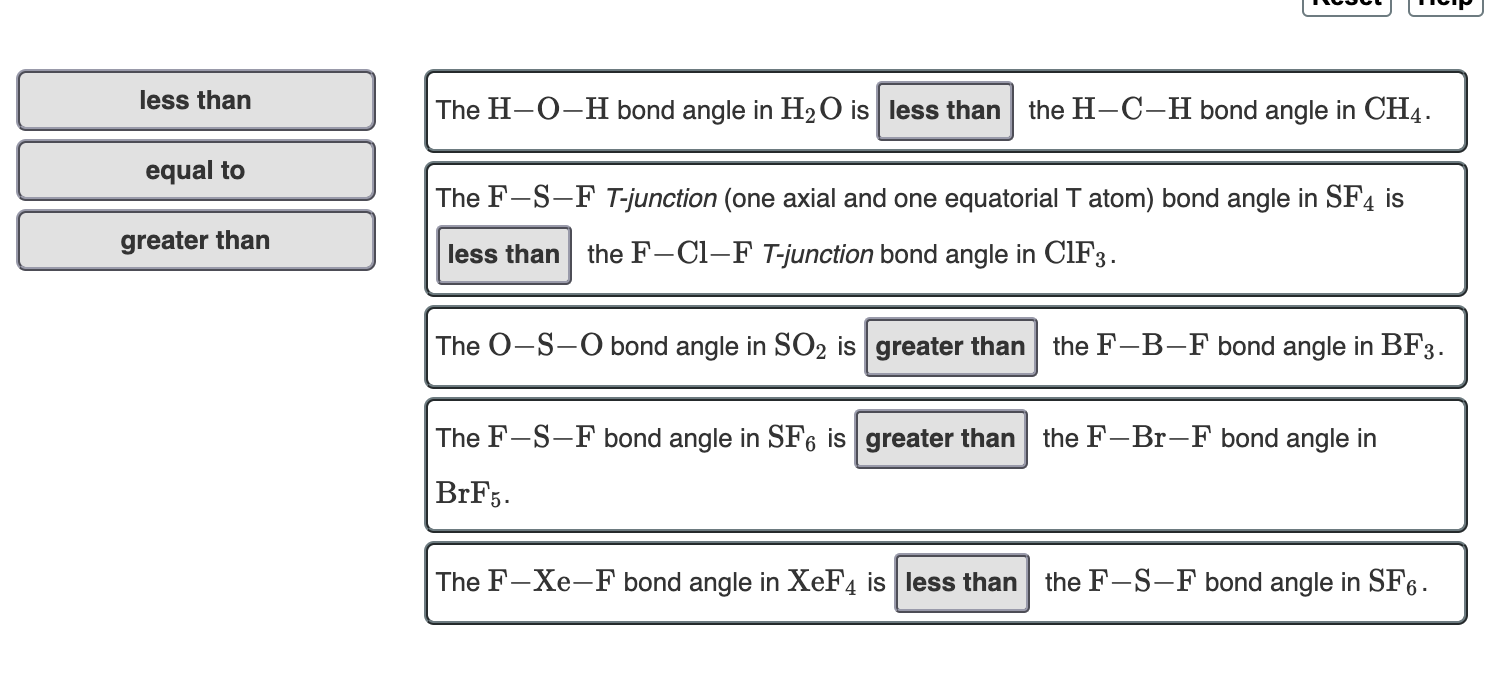
Solved Go 1 For The Water Molecule Oxygen Is More Chegg Com

Alcohols Advanced Scribble Notes Middle School Science Teacher Middle School Science Classroom Chemistry Worksheets
Comments
Post a Comment